Rank the solutions in order of decreasing H3O+ concentration, a crucial concept in understanding acid-base chemistry. This guide delves into the relationship between acid strength and H3O+ concentration, exploring pH and H3O+ concentration, the common ion effect, salt hydrolysis, and dilution’s impact on H3O+ concentration.
By unraveling these concepts, we gain a deeper comprehension of acid-base equilibria and their significance in various chemical processes.
1. Acid Strength and H3O+ Concentration
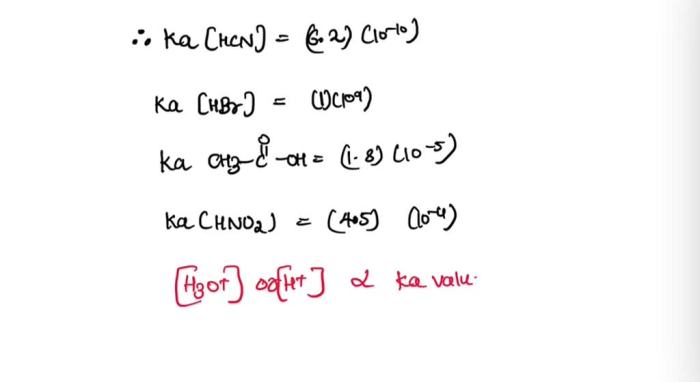
Acid strength is directly related to H3O+ concentration. Stronger acids dissociate more completely in water, releasing more H3O+ ions and resulting in a higher H3O+ concentration.
For example, hydrochloric acid (HCl) is a strong acid that dissociates almost completely in water, resulting in a high H3O+ concentration. On the other hand, acetic acid (CH3COOH) is a weak acid that dissociates only partially in water, resulting in a lower H3O+ concentration.
2. pH and H3O+ Concentration
pH is a measure of the acidity or alkalinity of a solution. It is defined as the negative logarithm (base 10) of the H3O+ concentration.
The logarithmic relationship between pH and H3O+ concentration means that a small change in pH corresponds to a significant change in H3O+ concentration. For example, a decrease in pH from 7 to 6 corresponds to a tenfold increase in H3O+ concentration.
3. Common Ion Effect
The common ion effect is the suppression of the ionization of a weak acid by the addition of a common ion. A common ion is an ion that is present in both the weak acid and the added salt.
For example, if sodium acetate (CH3COONa) is added to a solution of acetic acid (CH3COOH), the acetate ions from the salt will compete with the acetate ions from the acid for H3O+ ions, reducing the ionization of the acid and lowering the H3O+ concentration.
4. Salt Hydrolysis: Rank The Solutions In Order Of Decreasing H3o+
Salt hydrolysis is the reaction of a salt with water to produce an acidic, basic, or neutral solution. The type of hydrolysis depends on the nature of the salt.
For example, the hydrolysis of sodium acetate (CH3COONa) produces a basic solution because the acetate ion is a weak base. On the other hand, the hydrolysis of ammonium chloride (NH4Cl) produces an acidic solution because the ammonium ion is a weak acid.
5. Dilution and H3O+ Concentration
Dilution is the process of adding more solvent to a solution. When a solution is diluted, the H3O+ concentration decreases because the H3O+ ions are dispersed over a larger volume.
The mathematical relationship between dilution and H3O+ concentration is given by the following equation:
M1V1 = M2V2
where M1 and V1 are the initial molarity and volume of the solution, and M2 and V2 are the final molarity and volume of the solution after dilution.
General Inquiries
What is the relationship between acid strength and H3O+ concentration?
Stronger acids dissociate more completely in water, resulting in higher H3O+ concentrations.
How does dilution affect H3O+ concentration?
Dilution decreases H3O+ concentration by reducing the number of ions per unit volume.
What is the common ion effect?
The presence of a common ion in solution suppresses the ionization of a weak acid, reducing H3O+ concentration.